To Connect Via Zoom Click Here, Or Copy Link Below:
https://columbiauniversity.zoom.us/j/95048952569
EPR Studies of the Enzymatic Synthesis of the Organometallic H-Cluster of [FeFe] Hydrogenase
Presented by Prof. R. David Britt
Hosted by Prof. McDermott
Bio:
R. David Britt studied Physics at North Carolina State University (B.S) and the University of California Berkeley (Ph.D). While at Berkeley, his interests turned towards experimental biophysics, and he joined Melvin P. Klein’s laboratory in the Melvin Calvin Laboratory for Chemical Biodynamics in order to study photosynthesis. At the time the structure and function of the Mn-containing oxygen evolving cluster (OEC) of Photosystem II was poorly understood. David build a pulse EPR spectrometer (well before these were commercially available) and used it in the first high resolution EPR studies of this important catalytic center. In his new faculty position in the Chemistry Department at UC Davis, David and his students built a second- generation pulse EPR instrument that they employed in further studies of the OEC, for example revealing the 3+1 “dangler Mn” model. Over time, David and his group expanded their focus to a much broader array of enzymatic targets, including the [Fe-Fe] hydrogenase organometallic catalytic cluster which he discusses in his lecture here at Columbia, with bio-energy and bio- inspired synthetic catalysts remaining a prime interest. David is currently a Distinguished Professor and the Winston Ko Chair in Science Leadership at UC Davis. He is a Fellow of the International EPR Society (IES), the International Society of Magnetic Resonance (ISMAR), the American Association for the Advancement of Science, and the Royal Society of Chemistry, and has been awarded the Gold Medal of the EIS, the Zavoisky Award of the Russian Academy of Sciences, and the Bruker Prize of the Royal Society of Chemistry. He has also received the Bioinorganic Chemistry Award from the Royal Society of Chemistry and is this year’s winner of the Alfred Bader Award in Bioinorganic or Bioorganic Chemistry from the American Chemical Society.
Abstract:
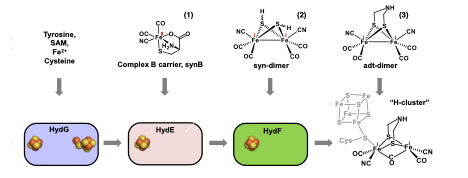
The [FeFe] hydrogenase enzymes are well suited to H2 formation, producing up to 10000 H2 molecules per second, and have therefore generated much interest for renewable energy applications. The H-cluster consists of a binuclear [2Fe]H subcluster which is linked via a bridging cysteine to a [4Fe-4S]H cluster. This [2Fe] H subcluster contains the organometallic elements of the H-cluster: the two irons each have a CO and a CN - terminal ligand and are bridged by a third CO and a unique SCH2NHCH2S azadithiolate (adt) moiety. The H+ and H2 substrates are proposed to bind to and react at this [2Fe]H unit. In addition to the relative rarity of enzymes carrying out organometallic reactions, biosynthesis of the H-cluster poises some specific challenges. Of course, free CO and CN - molecules are toxic. In addition, the bridging adt moiety is known to be unstable in solution. The H-cluster biosynthesis is performed by a set of three “maturase” proteins, HydE, HydF, and HydG, each containing Fe-S clusters. Two of these, HydE and HydG, are members of the radical SAM superfamily of enzymes, while HydF is a GTPase. Our approach to developing a viable mechanistic proposal for H- cluster synthesis includes chemical biology techniques such as cell free synthesis, isotope sensitive spectroscopy such as electron paramagnetic resonance, and the use of synthetic clusters that can serve as functional substitutes for enzyme intermediates.